ps2 molecular geometry|bonding domain and nonbonding domain : Cebu Predicting Electron Pair Geometry and Molecular Structure. The following procedure uses VSEPR theory to determine the electron pair geometries and the molecular structures: Write the . Unity Completed Handyman Legend [v1.0.6] [SupaShinKen Studio] Thread starter BaasB; Start date Feb 27, 2023; Tags 2dcg ahegao animated big ass big tits cheating corruption creampie exhibitionism footjob groping handjob lactation male protagonist milf oral sex point & click sandbox teasing vaginal sex voyeurism;
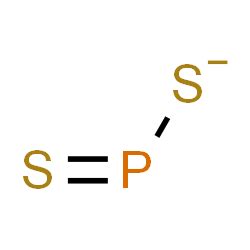
ps2 molecular geometry,Simple. Structure. Advanced. History. Comment on. this record. 3D. dithiophosphinate. Molecular Formula PS. Average mass 95.104 Da. Monoisotopic mass 94.918449 Da. ChemSpider ID 13406345. - Charge. More details: Names. Properties. Searches. .
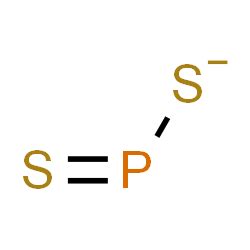
Molecular Geometry. After calculating the electronic geometry from VESPR we can determine the molecular geometry based on the bonding orbitals. If there are no lone .
D With two nuclei around the central atom and one lone pair of electrons, the molecular geometry of SnCl 2 is bent, like SO 2, but with a Cl–Sn–Cl bond angle of 95°. The molecular geometry can be described as a . Predicting Electron Pair Geometry and Molecular Structure. The following procedure uses VSEPR theory to determine the electron pair geometries and the molecular structures: Write the .
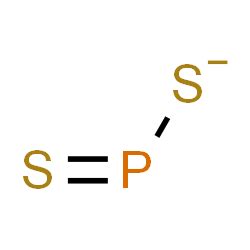
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other .Molecular Geometry. The specific three dimensional arrangement of atoms in molecules is referred to as molecular geometry. We also define molecular .
Sp² Hybridization, Bond Angle and Geometry (includes video) Sp Hybridization, Bond Angle and Geometry. Hybridization Shortcut. Sp³d and sp³d² Hybridization – An Overview. So let’s dig in. In addition .Problem sets built by lead tutors Expert video explanations. Go to Practice. Determine the molecular geometry and sketch each molecule or ion using the bond conventions .
The equilibrium geometry of the ground electronic state of PS2 are calculated using B3LYP, B3P86, CCSD (T), and QCISD (T) methods with 6-311G** and cc-pVTZ .Molecular geometry - The study of the three-dimensional arrangement of the atoms that constitute a molecule is called Molecular geometry. The molecular geometry can be determined by various spectroscopic methods and diffraction methods. To learn more about the Definition, Determination, Types, VSEPR theory and FAQs on molecular geometry, .
H 2 O Hybridization and Geometry. This concept of molecular vs electronic geometry changes even more when the molecule in question, while still sp³, has 2 lone pairs and therefore only 2 bonds. The water molecule features a central oxygen atom with 6 valence electrons. O = [He] 2s² 2p 4Molecular Geometry (11/16-18/2020) How can molecular shapes be predicted using the VSEPR theory? CHEM1.PS2.1 Draw, identify, and contrast graphical representations of chemical bonds (ionic, covalent, and metallic) based on chemical formulas. Construct and communicate explanations to show that atoms combine by transferring or sharing .Geometry of the water molecule with values for O-H bond length and for H-O-H bond angle between two bonds. Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule.It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters .ps2 molecular geometryD With two nuclei around the central atom and one lone pair of electrons, the molecular geometry of SnCl 2 is bent, like SO 2, but with a Cl–Sn–Cl bond angle of 95°. The molecular geometry can be described as a trigonal planar arrangement with one vertex missing. Exercise. Predict the molecular geometry of each molecule. SO 3; XeF 4 .
Use the Molecule Shape simulator to build a molecule. Starting with the central atom, click on the double bond to add one double bond. Then add one single bond and one lone pair. Rotate the molecule to observe the complete geometry. Name the electron group geometry and molecular structure and predict the bond angle.Figure 5.2.2 5.2. 2: The BeF2 molecule adopts a linear structure in which the two bonds are as far apart as possible, on opposite sides of the Be atom. Figure 5.2.3 5.2. 3 illustrates this and other electron-pair geometries that minimize the repulsions among regions of high electron density (bonds and/or lone pairs). H2S Molecular geometry. Hybridization of the given molecule H2S is sp3; the Sulfur atom is in center bonding with two Hydrogen atoms forming the bond angle less than 180 degrees. According to the VSEPR theory, the lone pairs of electrons repel each other, but as the Sulfur atom is less electronegative, the bond angle decreases to 104.5 . 1. The central atom, beryllium, contributes two valence electrons, and each hydrogen atom contributes one. The Lewis electron structure is. 2. There are two electron groups around the central atom. We see from Figure 9.2 that the arrangement that minimizes repulsions places the groups 180° apart. 3.
Contributors. 10.4: Geometry and Molecular Polarity is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. VSEPR theory predicts the three-dimensional arrangement of atoms in a molecule. It states that valence electrons will assume an electron-pair geometry that minimizes repulsions between areas of .
ps2 molecular geometry bonding domain and nonbonding domainContributors. 10.4: Geometry and Molecular Polarity is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. VSEPR theory predicts the three-dimensional arrangement of atoms in a molecule. It states that valence electrons will assume an electron-pair geometry that minimizes repulsions between areas of . SO42- Molecular Geometry. We can determine the molecular geometry of any given molecule using the VSEPR theory model and the AXN notation method. For example, for the Sulphate ion, the AXN notation would be AX4, as it forms bonds with four oxygen atoms. And as a result of this, it has a tetrahedral molecular geometry.A bond distance (or bond length) is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. Bond distances are measured in Ångstroms (1 Å = 10 –10 m) or picometers (1 pm = 10 –12 m, 100 pm = 1 Å). Figure \boldsymbol10.3.1 \boldsymbol 10.3. 1: Bond distances (lengths) and angles are shown for the .bonding domain and nonbonding domainThe molecular geometry of sulfur dioxide is a bent shape. Sulfur to the Oxygen ratio in Sulfur dioxide is 1:2. Sulfur dioxide molecule has two double bonds between the Sulfur atom and Oxygen atoms. There are 5 lone pairs of electrons in the molecule of SO2. Molar mass of sulfur dioxide = 64.066 g/mol. OpenStax. A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electrostatic force of attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction. All bonds can be explained by quantum .This online quiz is intended to give you extra practice in identifying the molecular and electron geometry of chemical compounds using VSEPR theory. Select your preferences below and click 'Start' to give it a try! Number of problems: 1. 5. 10. 25. 50. Question types (select at least one):
e) Determine the polarity of the molecule/ion. f) Again, take your time. Examine the shape of the model and see how the terms used to describe the electron and molecular geometry of the molecule/ion correspond with the model’s shape. See how the shape helps you decide on the polarity of the molecule/ion. 5. Repeat for each assigned molecule .
Ozone is one of the most common examples used to study the Lewis structure. The molecule of Ozone has three oxygen atoms. It is written as O3 in the core chemistry equations. To understand the hybridization, polarity and molecular geometry of the Ozone molecule it is crucial to know the Lewis structure of the same. Name of . The molecular geometry of any molecule depends on its Lewis structure, the arrangement of atoms, and its electrons. In an H2O molecule, the Oxygen atom forms two single sigma bonds with Hydrogen atoms. Although these two Hydrogen atoms are arranged symmetrically in the plane, the two lone pairs of electrons on the Oxygen atom .
ps2 molecular geometry|bonding domain and nonbonding domain
PH0 · ps2 lewis structure
PH1 · molecular geometry explained
PH2 · equatorial bonds molecular geometry
PH3 · electron geometry molecular geometry
PH4 · electron domain and molecular geometries
PH5 · determining molecular geometry
PH6 · bonding domain and nonbonding domain
PH7 · Iba pa
PH8 · 5 electron domains